Authors: Sophie Tissot, Jordan Meliani, Matthew Chee, Aurora M. Nedelcu, Justine Boutry, Jácint Tökölyi, Rodrigo Hamede, Benjamin Roche, Beata Ujvari, Frédéric Thomas & Antoine M. Dujon
Source: Scientific Reports (Sep 2024)
Abstract
Recent theoretical advances in the One Health approach have suggested that cancer pathologies should be given greater consideration, as cancers often render their hosts more vulnerable to infectious agents, which could turn them into super spreaders within ecosystems. Although biologically plausible, this hypothesis has not yet been validated experimentally.
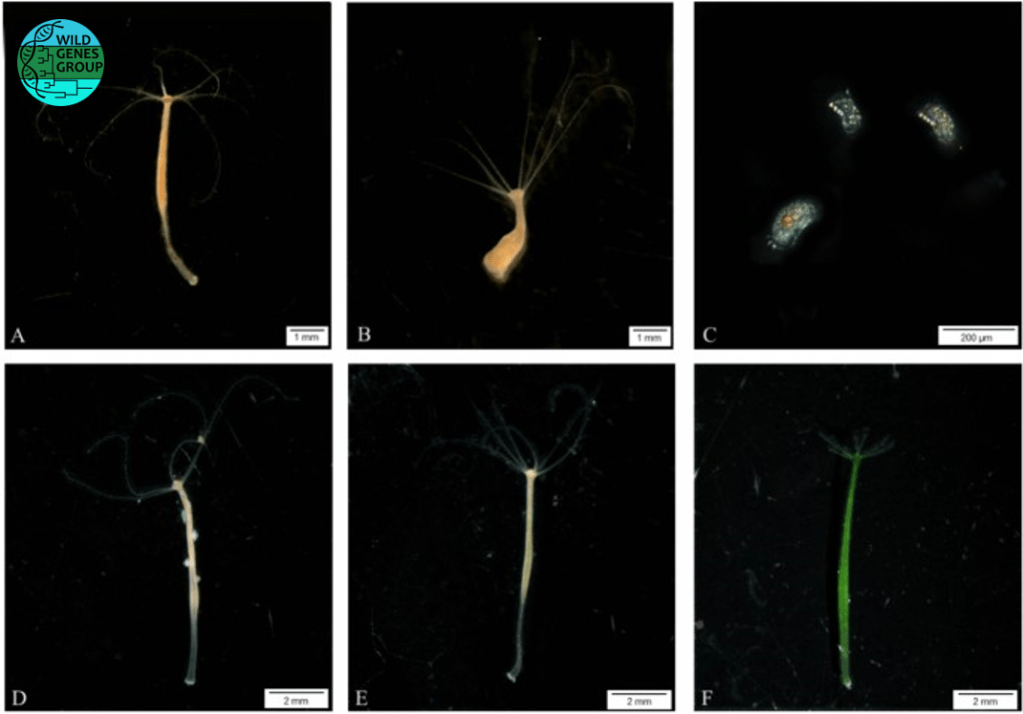
Using a community of cnidarians of the Hydra genus (Hydra oligactis, Hydra viridissima, Hydra vulgaris) and a commensal ciliate species (Kerona pediculus) that colonizes them, we tested whether tumoral polyps of H. oligactis, compared to healthy ones, played an amplifying role in the number of ciliates, potentially resulting in a higher likelihood of infection for other community members through spillovers. Our results indicate that K. pediculus has a higher proliferation rate on tumoral polyps of H. oligactis than on healthy ones, which results in the infestation of other hydras. However, the magnitude of the spillover differed between recipient species.
This study provides to our knowledge the first elements of proof of concept that tumoral individuals in communities could act as super spreaders of symbionts within and between species, and thus affect biotic interactions and dynamics in ecosystems.