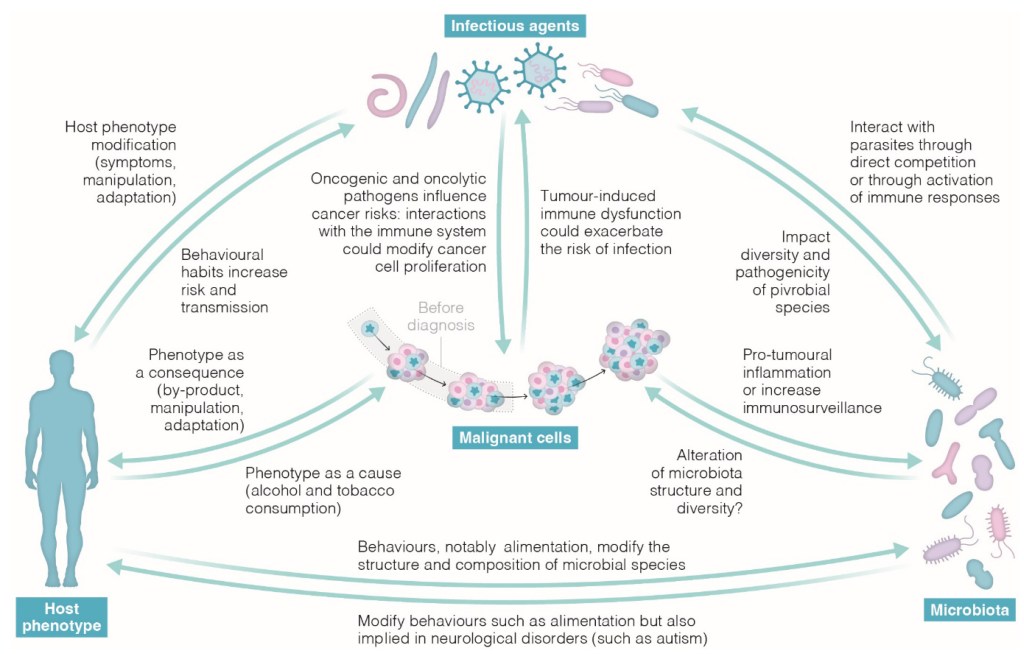
Authors: Audrey Arnal, Erika Burioli, Lisa Jacquin, Sophie Labrut, Stéphane Duchon, Marie Rossignol, Delphine Nicolas, Beata Ujvari, Antoine Dujon, Jordan Meliani, Jérôme Abadie, Frédéric Thomas, Vincent Corbel
Source: Ecotoxicology and Environmental Safety (Aug 2025)
Abstract
Malathion is a widely used pesticide with potentially oncogenic properties and may have deleterious effects on organism health and fitness. Although malathion use is now restricted in the European Union, it remains widely used for public health campaigns in other parts of the world, particularly for mosquito control. Understanding its sublethal and long-term effects is thus essential, both for evaluating its ecotoxicological impacts and for anticipating resistance mechanisms. However, empiric data on its effects in wild organisms – especially in invertebrates – remain limited.
Here, we quantitatively investigated whether larval exposure to environmentally realistic concentrations of malathion could affect mosquito tissue structure and gene expression profiles of adult Aedes aegypti (yellow fever mosquitoes), using both RNA-seq and histological approaches.
Results show no neoplastic or pre-neoplastic lesions in adults exposed to malathion during larval development, contrary to previous studies in other organisms showing carcinogenic effects of malathion. However, our differential gene expression analyses revealed significant changes in genes related to mitochondrial function, energy metabolism, and detoxification pathways, suggesting significant physiological impacts of malathion in adults after early-life pesticide exposure.
Notably, females exhibited stronger transcriptomic responses than males, including the upregulation of genes involved in detoxification (e.g., P450 cytochromes), olfactory perception, and stress response, with potential consequences for resistance mechanisms.
Our findings underscore the ability of mosquitoes to mount transient molecular responses to environmental pollutants, potentially contributing to the long-term selection of metabolic resistance traits – an outcome with important implications for vector control strategies.